Model 8012, KN95 Respirator, 4-Ply (US FDA Non-NIOSH Authorized)
Product Information
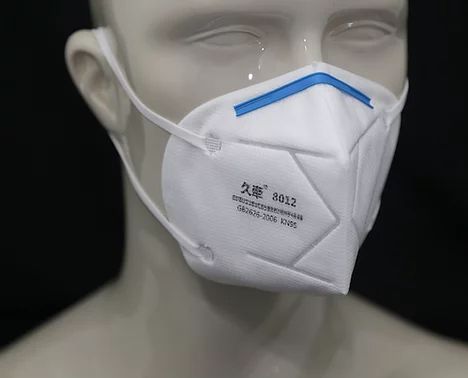
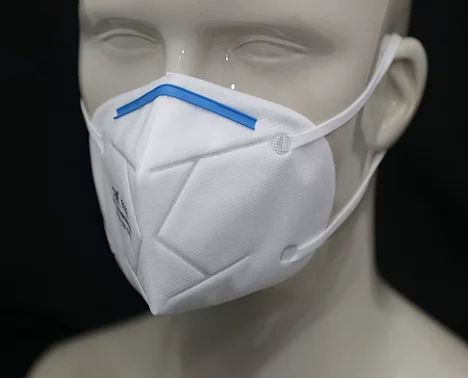
This mask is rated KN95 against China GB2626-2006 Standard. It has a filtering efficiency of ≥95% for non-oily particulate matter. It has a flat-folded design which makes it easy to carry.
It is made up of four layers of material: SBPP-Static Control Filter-MBPP-SBPP. The static control filter material helps block particulates in the air. The mask also comes with ultrasonically welded earloop and a bendable nose clip that improves the sealability of the respirator on the face.
Mask-KN95-8012 is US FDA authorized non-NIOSH approved disposable filtering facepiece respirator for Covid-19 pandemic respirator shortages.
It is made up of four layers of material: SBPP-Static Control Filter-MBPP-SBPP. The static control filter material helps block particulates in the air. The mask also comes with ultrasonically welded earloop and a bendable nose clip that improves the sealability of the respirator on the face.
Mask-KN95-8012 is US FDA authorized non-NIOSH approved disposable filtering facepiece respirator for Covid-19 pandemic respirator shortages.
Product Features
- Mask has effective filtering non-oily particulate matter, filtering efficiency: ≥ 95%
- Constructed with 4 layers of material: Inner and outer layers of Spunbond Polypropylene (SBPP), middle layer of an electrostatic filter material and another inner layer of Meltblown Polypropylene (MBPP)
- Has a white earloop and a bendable nose clip to improve sealabilty of the respirator on the face
- Folded design makes it easy to carry
- Rated KN95 against China's GB2626-2006 standard
Order Information
Model
Mask-KN95-8012
Description
7.5 kg/case, 51cm x 46cm x 36cm
Pcs/Box
30
Boxes/Case
24